Our Team

Marina Felt
Biomedical Engineering
Marina is a 5th year biomedical engineering major from Boulder, CO. Throughout her time at Cal Poly she has focused on cardiovascular and neurovascular devices. Following graduation she plans to pursue a career as an R&D engineer on a neurovascular stent team.

Blake Juett
Biomedial engineering
Blake Juett is a 4th year biomedical engineering major from Los Angeles County, CA. He has spent his time working on projects relating to prosthetics and orthotics. Following graduations he plans to work in the medical device industry.

Arshia Nazearni Houshmand
Biomedical engineering
Arshia is a 5th year biomedical engineering major from Orange County, CA. Since transferring to Cal Poly in 2019, he has been focusing his time towards medical device manufacturing and design. Following graduation he plans to pursue a career within a medical device company.
Project Video
Our Project's Digital Poster
Problem Statement
limitations of current devices
Majority of tourniquet systems today require manual application of pressure which can result in inadequate pressure, or failure to apply the system correctly, especially in emergency situations. These tourniquets also require periodic monitoring throughout the duration of it’s application. Additionally, manual tourniquet application often result in an applied pressure that is must higher than the minimum occlusion pressure, this can result in post tourniquet syndrome, and permanent nerve and muscle damage.
solution
This project will create an automatic tourniquet system that minimizes user effort while consistently providing sufficient pressure to stop bleeding in a time-efficient manner. The device will address the limitations of current products, and must follow military protocol and be designed to be easily attached to military equipment. Additionally, the tourniquet will be designed to be consumer-friendly with the possibility of being applied to household and medical-setting emergencies with the primary focus being the U.S. military.
Indications For Use
A. For military personnel over 16 years old
B. To be applied for less than 2 hours
C. Tourniquet is to be placed around the area by trained personnel, then the system automatically applies pressure
D. Blood pressure and time of application will be reported to medical personnel
Design Concepts
Design concepts of the automatic tourniquet were generated using morphology tables and introductory sketches. Using pugh matrices, the team was able to select the design concept that would provide the most success during the prototyping stage and could provide reliable results.
Due to the limited-time nature of this senior project course, the tourniquet was not designed to be in an internal system that would contain all of the components. In the ideal scenario, our design would consist of an all-in-one tourniquet that contains the electronics and tourniquet that would be attached together. As a result of the limitation, the team decided to move towards an external system wherein each individual part of the system was split. In doing so, the team will have the ability to rapidly prototype and test our design without constraining to a specific size or dimension.
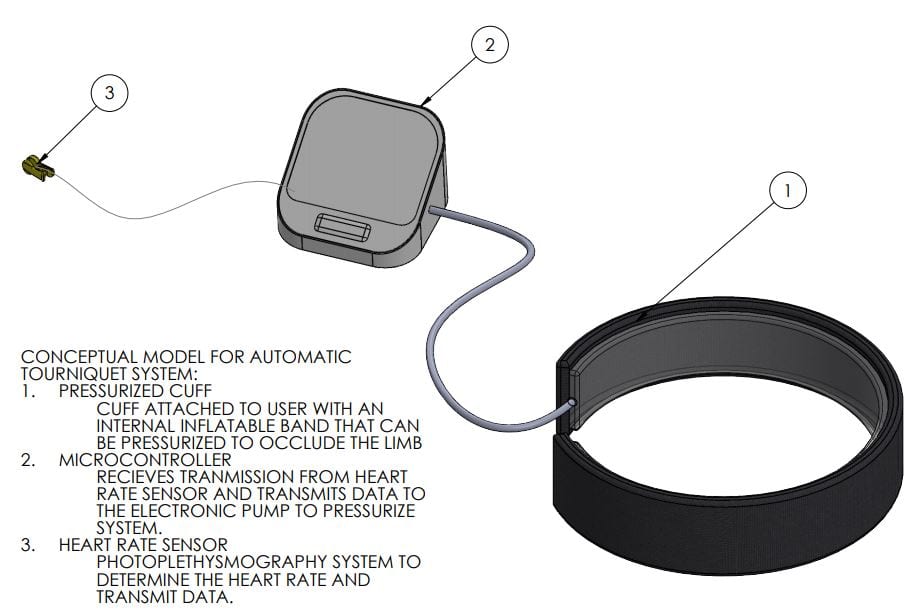
Manufacturing Process
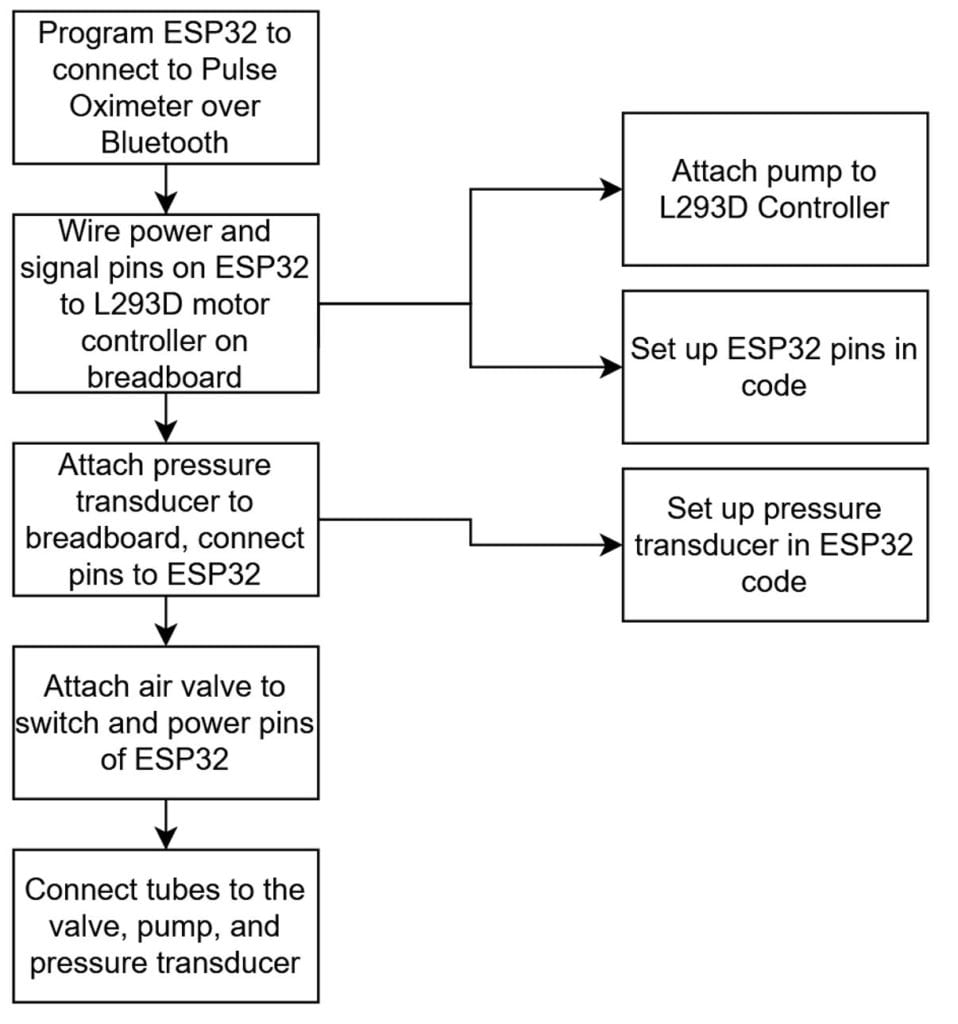
Shown above is our manufacturing process instruction plan. Since we currently use mostly pre-existing components, manufacturing involves connecting these components together and properly programming the ESP32 board.
Software Componenets
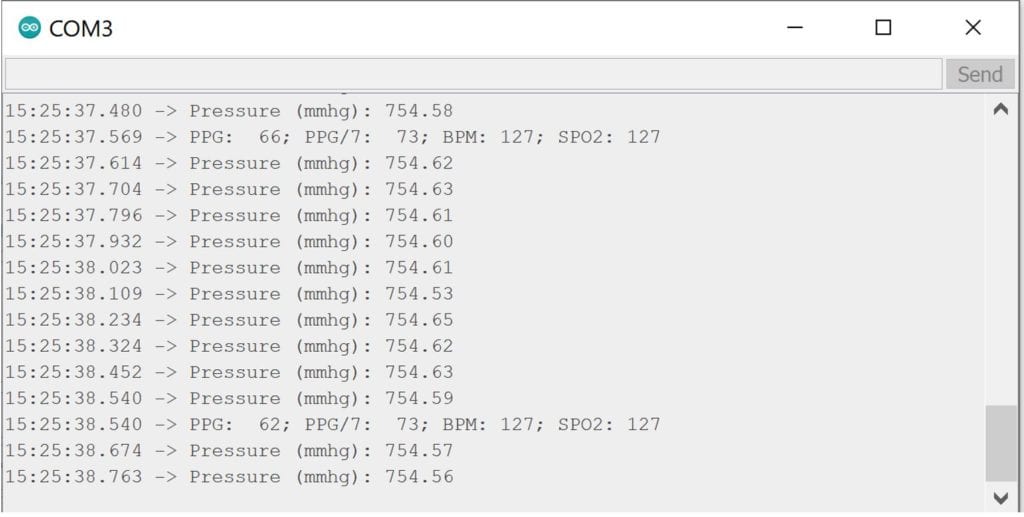
The photo above shows the pressure and pulse oximeter output. The oximeter output most relevant to this project is the beats per minute (BPM).
Our software is loaded onto an ESP32 board, which acts as the main controller of our system. This board is programmed to wirelessly connect to a pulse oximeter and read the heart rate data. Using this data, as well as data from the pressure transducer, the board then tells the air pump to start inflating the tourniquet. Once a pulse is no longer detected or the pressure reaches a cutoff value, the motors will stop pumping air in. Until the tourniquet is ready to be taken off, the system can correct for any air loss. if pressure falls or heart rate is detected again, the pump will turn back on to reinflate the tourniquet. When the tourniquet is ready to be taken off, a switch can be turned on which opens an air valve that lets air flow out of the system.

California Polytechnic State University
Department of Biomedical Engineering
Engineering Specifications
- Constricts blood flow
- Complete occlusion of vasculature at attachment site resulting restricted blood flow distal to the attachment site.
- Speed of pressurization
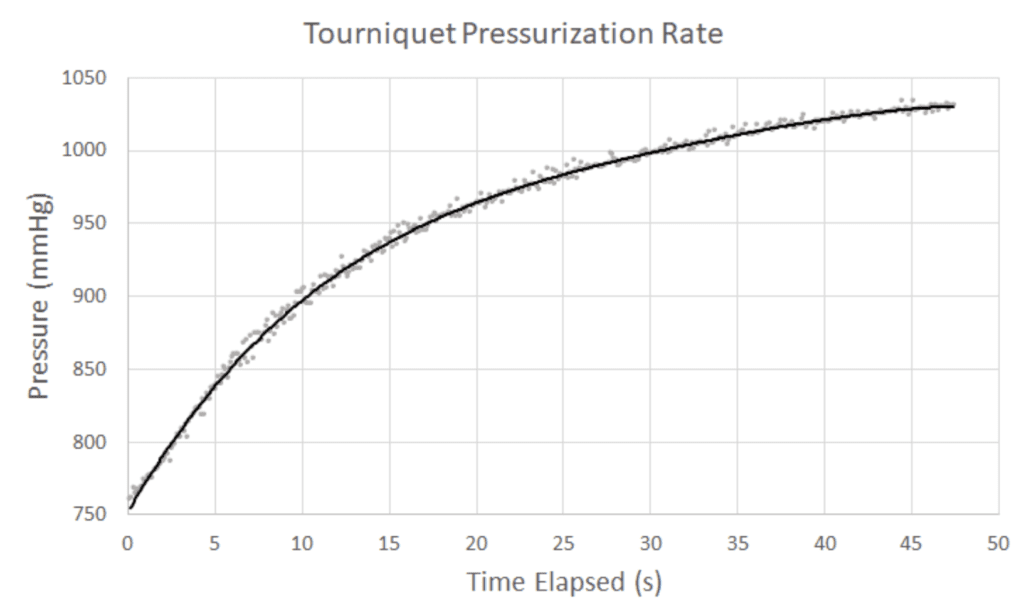
- System must pressurize at a minimum rate of 10 mmHg/s
- Time of complete pressurization/ depressurization
- Total time for pressurization must be under 45 seconds
- Total time for depressurization must be under 10 seconds.
- Accuracy of pressurization
- Pressure within the system must be accurately read within 5% standard error.
- Maximum pressure
- The device must be capable of reaching a maximum pressure of 350 mmHg.
- Sustains pressure over time.
- Pressurized tourniquet must sustain pressure for a minimum of two hours.
- Different arm size compatibility
- The device must be adjustable for a range of arm circumference.
Design Verification & Testing
Test plan Summary
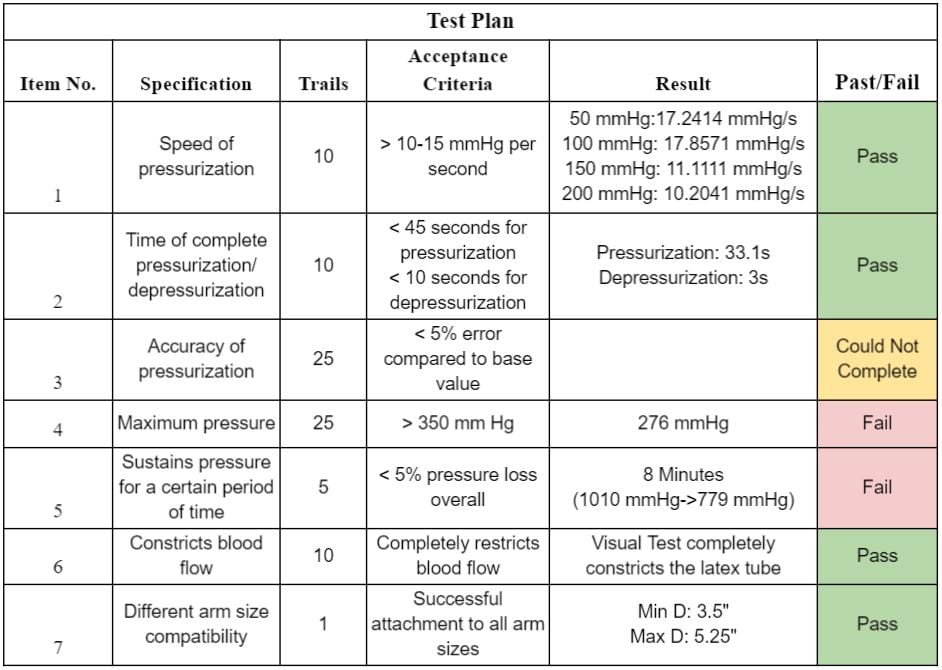
Test results
Speed of Pressurization- The pressurization rates were calculated at 50 mmHg intervals until 200 mmHg. System passed at each interval.
Time to Complete Pressurization/Depressurization-The time to reach a sufficient occlusion pressure (250mmHg) and depressurize was measured with a timer. System passed for pressurization and depressurization.
Accuracy of Pressure- The test plan for accuracy requires a manual pressure bulb to manually measure the internal pressure to be compared to the data recorded by the pressure transducer. Given more time, and the purchase of this component, this test can be conducted.
Max Pressure – Device was allowed to pressurize until a maximum pressure was reached. System failed. The pumps were capable of reaching the max pressure when directly connected to a power source, however, when connected to system the maximum pressure in the system was not reached.
Time to Sustain Pressure – Device was run until a sufficient occlusion pressure was reached (250mmHg), and shut off/closed to maintain the pressure. System failed. The system dissipates pressure overtime – reaching atmospheric pressure in 8 minutes, indicating an air leak within the system.
Constricts Blood Flow – Device was connected to testing apparatus and pressurized to occlusion pressure. The percent occlusion of the latex tube (vasculature) was measured. System passed.
Arm Size Compatibility – The range of diameters the tourniquet cuff was compatible with was measured. System Passed.
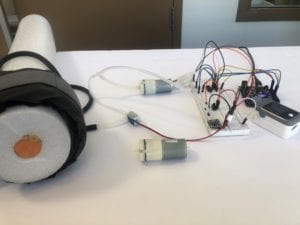
Testing apparatus

Frontal view of testing apparatus.

Isometric view of testing apparatus.
Final Design
Component Break-Down
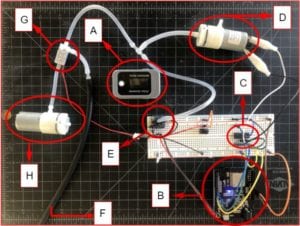
- [A] Pulse Oximeter (sends PPG pulse data via Bluetooth)
- [B] ESP 32 Arduino (receives pulse data, coded to initiate all system responses and record feedback)
- [C] Microcontroller (coded to power air-pump when there is a detectable pulse)
- [D] Pump 1 (pressurizes system)
- [E] Pressure transducer (measures and records systemic pressure)
- [F] Tourniquet (connection to tourniquet cuff for pressurization)
- [G] Electronic Valve (manipulates direction of flow for pressurization and for depressurization)
- [H] Pump 2 (Vacuum configuration, depressurizes the system)
The final prototype shows successful design and integration of the three initial components necessary for device function (heart rate sensor, coded feedback control system, and a pneumatic tourniquet cuff). The system uses data gathered from a pulse oximeter for user heart rate values, this is done using photodiodes and PPG technology. The control system assesses whether or not there is a detectable pulse distal to the cuff, and initiates/continues pressurization until there is no longer a detectable pulse. Finally, there is a switch incorporated into the design that will trigger device depressurization.
Limitations
Current limitations of the automatic tourniquet are based on the lack of additional testing required in order to obtain information that is necessary to create a fully functioning device. Our limitations are based on four main points:
A. Current pulse oximeter technology will have no readings when occlusion of the vein has gone lower than 20% pulsatile flow
B. The pressure value obtained from our pressure transducer must be verified against a doppler device
C. Pressure gradient of our cuff from one edge to another must be calculated in order to have an accurate depiction of the pressure within the cuff
D. Overlaps of the cuff, when applied to the limb, can create a combined pressure point that might be too high
Based on the four main limitations, our device would require additional testing and validation in order to proceed from prototype to functional device.
Future Improvements
Based on the final prototype that was designed for this project, our team implemented a list of future improvements that can be applied in order to obtain more reliable results and move towards a fully functional device. The list is as follows:
- A. In order to improve pressurization rates, an additional pump can be added to the system.
- B. Due to power draw issues currently within the system, the power input must be redesigned
- C. Design a printed circuit board (PCB) to consolidate system electronics
- D. Improve the fit between the pressure transducer and its tubing to reduce air leak and increase reading accuracy
- E. Add a digital screen for data output such as heart rate, current pressure, and other additional feedback as needed
- F. Implementation of a mechanical on/off switch within the system for emergencies
Conclusion
In conclusion, the automatic tourniquet system that was designed and created over the course of this senior project was successful. Although issues were encountered with the current design such as power draw issues and leakage of air, our team created a functional prototype that can be built upon in order to be marketable. Our device has many opportunities to grow and improve, and with that, our team hopes to sponsor the project for continuation by other students within Cal Poly – SLO.
Acknowledgements
We would like to thank our senior project advisor Dr. Michael Whitt, Dr. John F. Kragh, Dr. Christopher Heylman, the BME department, and the Hannah-Forbes Project Fund for their assistance.

