Our Team

Amanda Tsai
Amanda is a 4th year materials engineering undergraduate and polymers and coating science master’s student graduating June 2022 from Cal Poly. She is a member of the Honors Program on campus. Amanda is passionate about helping the environment, and is currently conducting research on a sustainable leather alternative with the Hamachi Group at Cal Poly. She also serves as the current Treasurer of Tau Beta Pi and President of Alpha Sigma Mu, two engineering honors societies on campus.

Vincent Guarino
Vincent is a Materials Engineering senior, graduating from Cal Poly this spring. Vincent has been working as a resident advisor in the College of Engineering’s on-campus residence hall since the start of his sophomore year and currently serves as the Vice President of Alpha Sigma Mu, the materials engineering honor society. Vincent performed past research on the suitability of rolled titanium coils for artificial reefs and wave energy dissipation. After graduation, he will begin his career working for Phillips 66 as a Mechanical Integrity Engineer.
Acknowledgements
We would like to thank our senior project advisor, Dr. Trevor Harding and our project sponsor, Meissner Filtration. This project would not have been possible without them.
Our Project's Digital Poster
Problem Statement
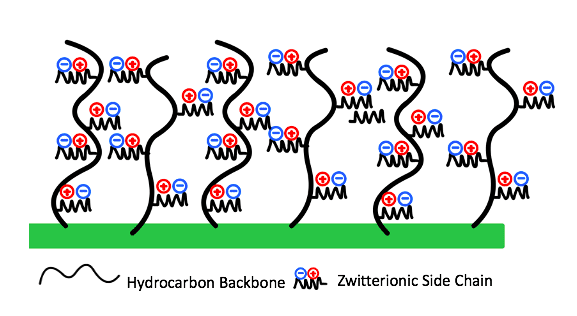
Meissner Filtration is a leader in microfiltration across a wide range of industries worldwide. As solutions are filtered through the polymer membranes, fouling will occur. Fouling, or the deposition or adsorption of particles onto a membrane’s surface can prevent the filtrate from flowing through the membrane, resulting in poor filter performance. Therefore, the goal of this project was to design a charged coating for a hydrophobic commercial membrane that optimizes non-specific antifouling performance (Fig.1).
Methods Overview
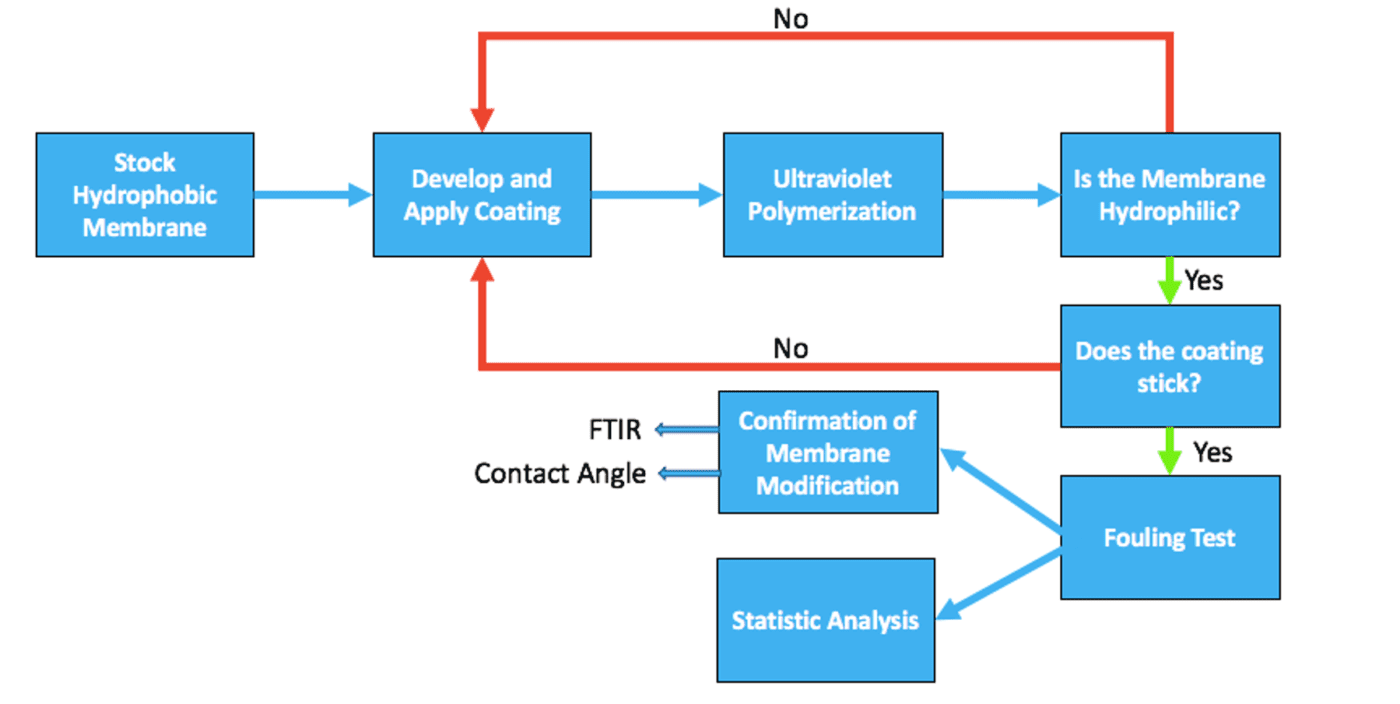
In the process of forming an antifouling filtration membrane, a commercial hydrophobic membrane was first obtained. Research was then conducted to determine the best approach for the surface charge modification of the commercial membrane. Various polymerization conditions were then tested to find the optimal UV polymerization time, monomer concentration, and initiator concentration. Rough hydrophilicity tests and water flow tests were used to confirm the durability of the coating during application. The antifouling performance of the final formulations were then tested using Ovaltine as a foulant. Three tests were conducted on each sample and the fouling performance of a commercial antifouling membrane was also measured for comparison. The membranes were then characterized by Fourier-Transform Infrared Spectroscopy (FTIR) and contact angle measurements to confirm that membrane modification occurred. Lastly, statistical analysis was used to determine which coating parameters result in the best antifouling performance. The steps for formulation, characterization, and fouling testing of a UV polymerizable, charged zwitterionic membrane coating are shown in Figure 2.
Formulation
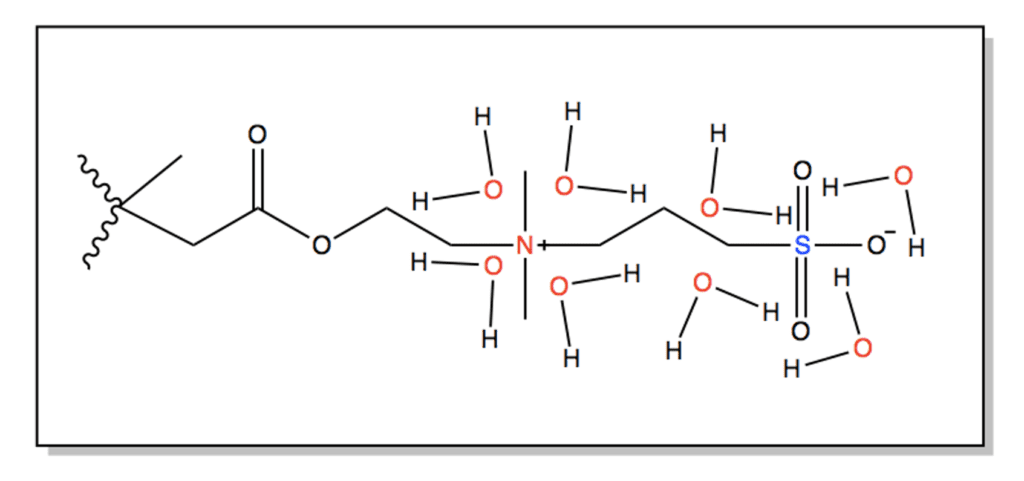
A commercial SBMA or sulfobetaine methacrylate monomer was originally chosen for a grafting from approach. SBMA is a zwitterionic material consisting of a positive functional group and a negative functional group resulting in an overall neutral charge. These charged groups can provide superior antifouling performance through mechanisms of hydration shell formation and steric hinderance.
Zwitterionic materials combine water molecules via electrostatic interactions, which work to form a hydration shell on the membrane’s surface.This hydration shell acts as a barrier that prevents the foulants from directly contacting the surface as the added hydrophilicity of the modified surface provides a higher Gibbs free energy, making it less likely for foulants to break through the hydration shell (Fig. 3).
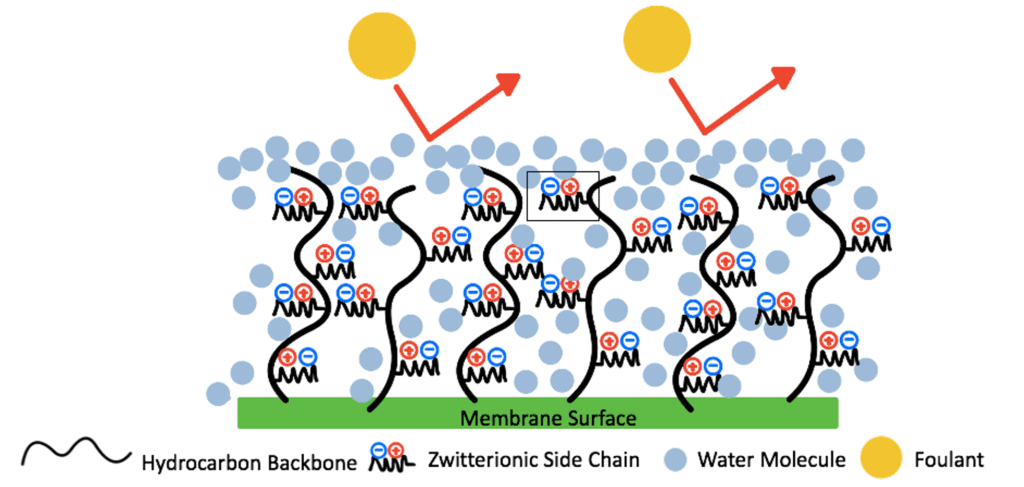
Zwitterionic materials are attractive as each repeat unit can integrate up to 8 water molecules creating a denser and thicker hydration shell than of other antifouling materials (Fig. 4).
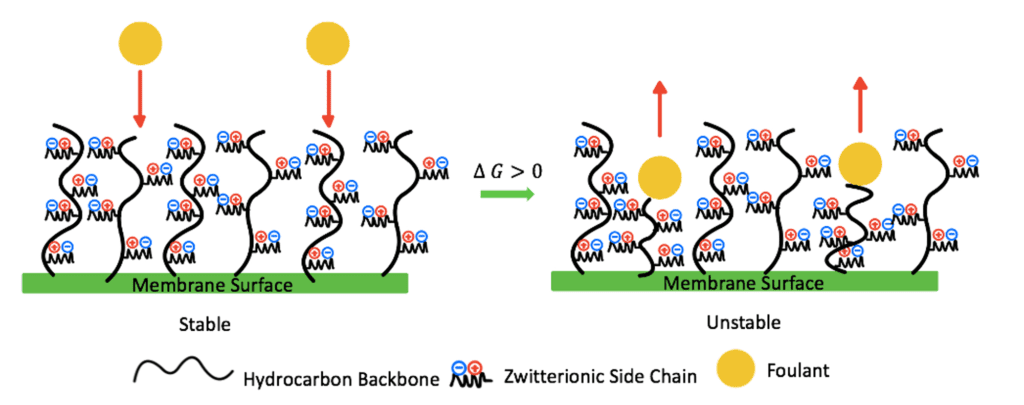
The antifouling properties of a zwitterionic coating are also controlled by the steric hindrance of the zwitterionic polymer chains. These chains have a large excluded volume. Therefore, as foulants contact the zwitterionic surface, the polymer chains will compress, increasing their Gibbs free energy, entering an unstable state (Fig.5). The polymer chains will later recover to their stable swelled state, preventing foulants from reaching the surface of the membrane.

This project is sponsored by Meissner Filtration.
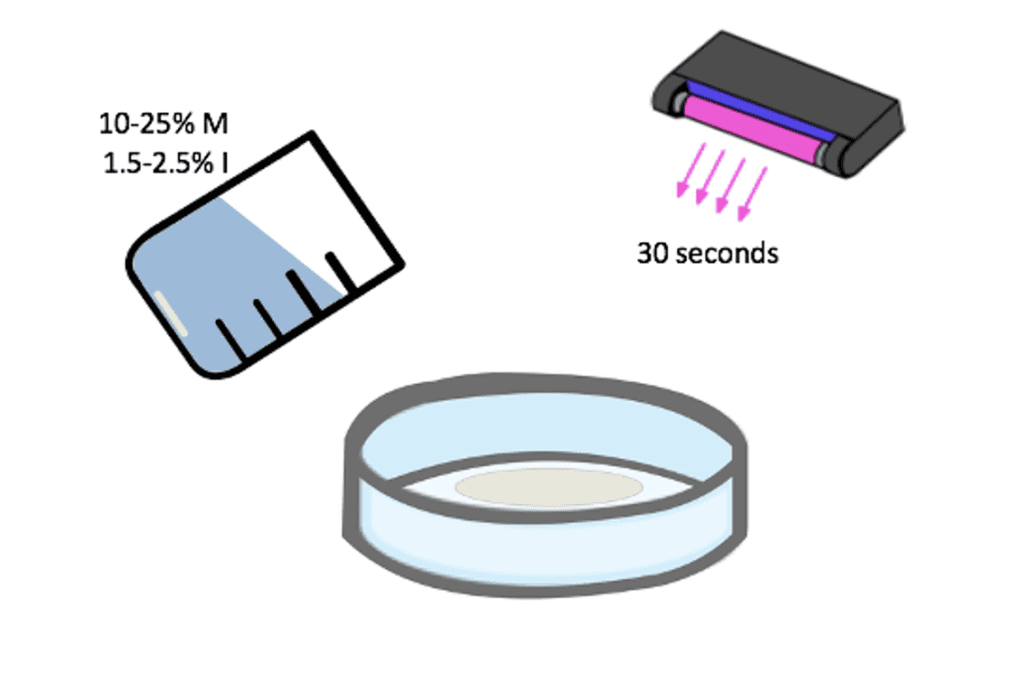
The basic formulation of the SBMA coating consisted of SBMA monomer and SpeedCure 4265 initiator dissolved in a 1:2 water and IPA solution. Various combinations of initiator concentration and monomer concentration were developed. The monomer concentration, initiator concentration, and UV exposure time was narrowed to 10-25% M, 1.5-2.5% I, and a 30 second polymerization time, respectively through the use of rough wetting tests and water flow tests (Fig. 6). 20% of the original monomer concentration was later replaced with ethylene glycol dimethacrylate, a crosslinker, due to a lack of adhesion between coating and the membrane. The addition of the crosslinker transitioned the polymerization process from being a grafting from approach to a mixture of grafting from and grafting to. The crosslinker helps increase adhesion by allowing the buildup of molecular weight in solution increasing the probability of covalent attachment of the polymer to the membrane.
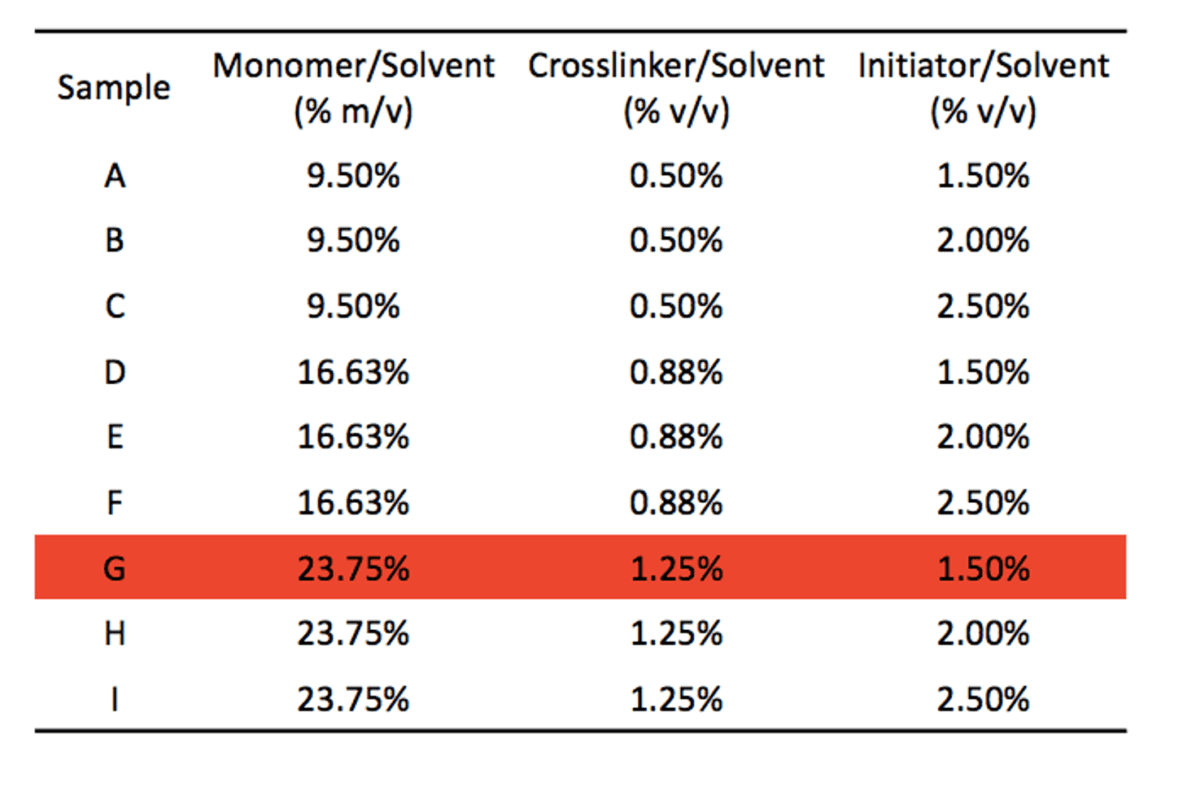
The final formulations are shown in the table below. Sample G was eliminated due to its incomplete wetting during the rough hydrophilicity test.
Characterization was then used to confirm membrane modification, and fouling tests were used to measure antifouling performance.
Characterization
ContAcT Angle MeasurEMENts
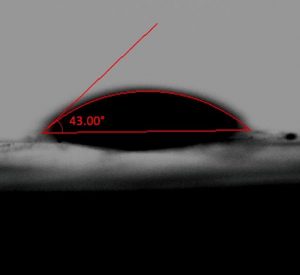
A goniometer was used to measure the contact angle of a water droplet on the unmodified and the modified membranes. Ten measurements were taken across the membrane surface for each formulation combination to ensure uniform hydrophobic-hydrophilic nature across the surface. The average water contact angle for the unmodified commercial membrane was determined to be above 90 degrees. Therefore, the unmodified membrane was confirmed to be hydrophobic (Fig. 7).

Meanwhile, all of the modified membranes tested were determined to be hydrophilic as they all had a contact angle below 90 degrees (Fig. 8). The measured change in the hydrophilicity confirmed that membrane modification occurred.
Fourier-Transform Infrared Spectroscopy
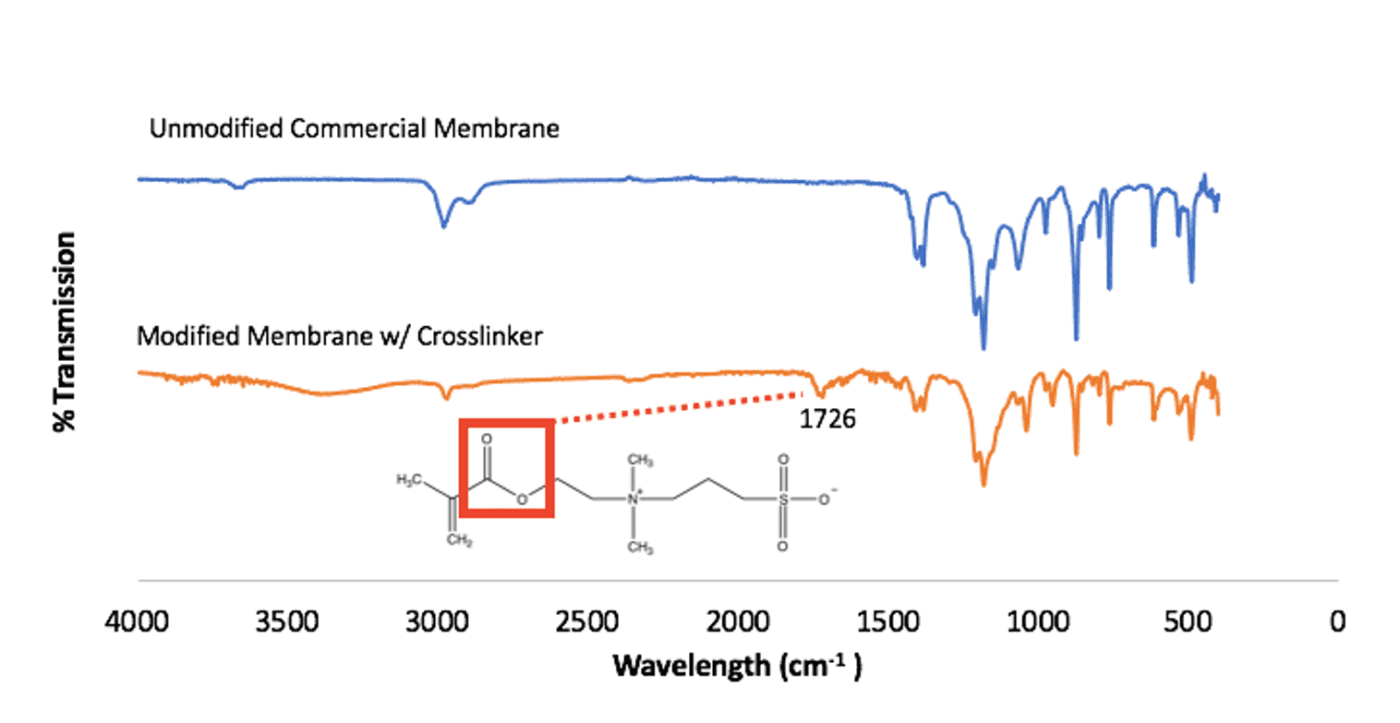
Fourier-transform infrared spectroscopy (FTIR) was collected for the unmodified commercial membrane and the modified membrane. A new peak is seen to emerge around 1730 cm^-1 in the modified membrane’s FTIR spectrum (Fig. 9).
This peak is representative of the ester group in the SBMA monomer structure. This difference between the two FTIR spectrum show that the polySBMA covalently bonded to the membrane surface, indicating that modification occurred.
Fouling Testing
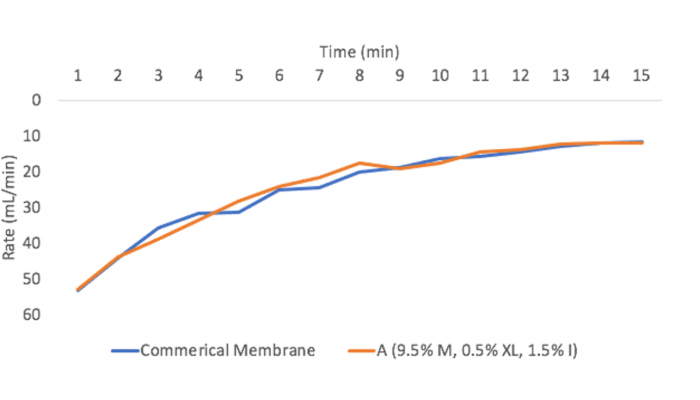
Fouling tests were then conducted on the remaining 8 membranes using a solution of .1 g/L Ovaltine in water solution, a procedure used to mimic fouling experienced in the biopharmaceutical industries. Three tests were conducted on each sample and the fouling performance of a commercial antifouling membrane was also measured for comparison. For the fouling test, the ovaltine solution was filtered at a pressure of 10 psi and the volume of the filtrate was measured every minute until the rate of the filtrate flow was less than 10 mL/min, a performance at which the membrane was determined to be unusable (Fig. 10).

Figure 11 shows the performance of one of the membranes we developed compared to a commercially available membrane. It can be determined that the fouling performance of membrane A was similar to that of the commercial membrane.
The table below shows the average volume of foulant collected after 15 minutes. A one way ANOVA was performed using the volume after 15 minutes.
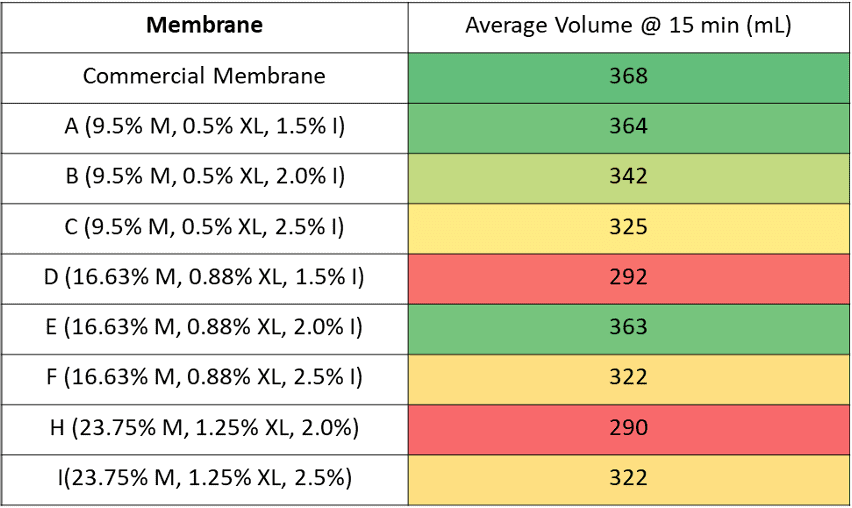
From the results of the ANOVA, it can be seen that the p value is 0.033. Since this value is less than 0.05, the difference between some of the membrane combinations’ flow rates are large enough to be statistically significant at a 95% confidence interval.
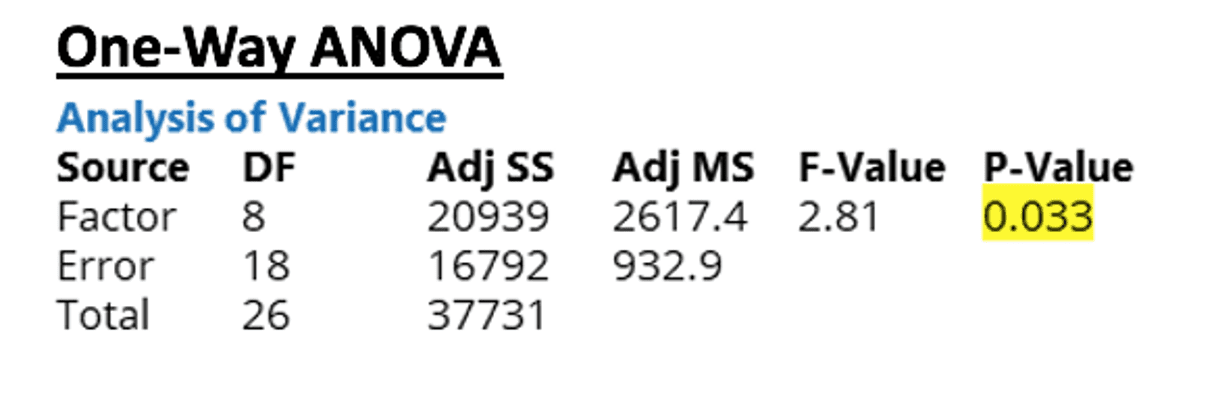
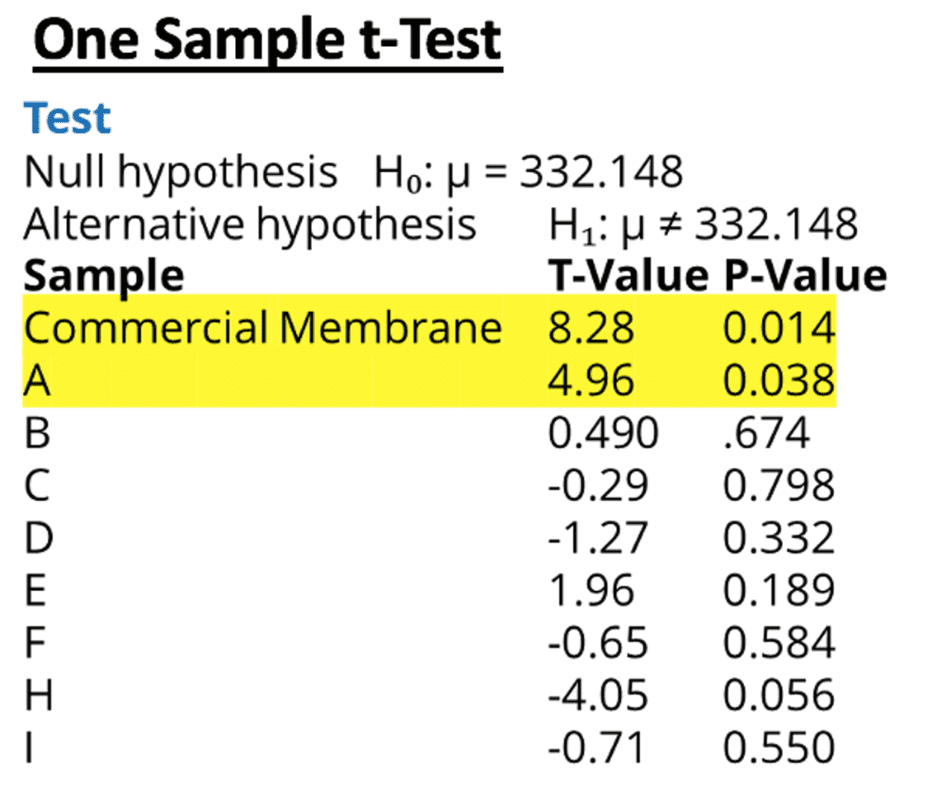
Tukey tests were used to determine which membranes differed; however, it indicated that none of the means were statistically different. These conflicting results indicate that the mean of one or more of the membranes is statistically different from the grand mean.
Therefore, a one-sample T test was performed for each sample using the grand mean as the hypothesized mean. Both the commercially available membrane and membrane A had p-values less than .05 indicating that these two samples had a average volume that was statistically different from the grand mean.
Conclusion
Findings
1) A zwitterionic monomer, such as SBMA, can be used to induce antifouling.
2) A coating with 9.5% monomer, 0.5% crosslinker, and 1.5% initiator concentration (Membrane A) had the best antifouling performance after the commercial membrane.
Future Research
1) Investigate solutions more dilute than Membrane A.
3) Elucidate the relationship of initiator composition and UV curing time.
4) Prepare a cost analysis of the Membrane A and compare to that for commercial membranes.

