about me

Joshua Laguio
Senior project lead
My name is Joshua Laguio (He/Him/His) and I am a fourth-year materials engineering student from Union City, CA. During my time in the MATE department, my interests grew towards electronic materials and devices as well as applications in the consumer electronics and semiconductor industry. In my free time, I enjoy trying new foods, watching anime, and hiking.
Connect with me on LinkedIn!
Acknowledgements
I would like to thank Professor Desalegn Mengistie for his time and efforts in supporting this senior project, Alison Chew and Jayden Wang for being time flexible and amazing in the lab, and Eric Beaton for providing the necessary materials and equipment to carry out the experiments.
Conducting Polymer-PVA Composite for Flexible Thermoelectrics
Background
Thermoelectric materials are materials with the ability to generate an electrical potential through a temperature gradient. The desired properties for a high performing thermoelectric material are high electrical conductivity, low thermal conductivity, and high Seebeck coefficient. The Seebeck coefficient is the measure of induced voltage in response to a temperature gradient. Typical inorganic semiconductors for thermoelectrics have several drawbacks including high cost, toxicity, difficult processability, and source scarcity. While, conducting polymers are relatively cheaper, mechanically flexible, low toxicity, easy to manufacture, and commercially available. The use of flexible thermoelectric materials widens the application of thermoelectric applications, especially in wearable technology.
Problem Statement
- Poly(3,4-ethylenedioxythiophene):polystyrene sulfornate (PEDOT:PSS) is a promising candidate for organic thermoelectrics due its high electrical conductivity, low thermal conductivity, and good film forming ability. However, it lacks the flexibility and stretchability for flexible technology. The goal of the project is to mix PEDOT:PSS with a flexible polymer and investigate the effect of varying ratios on the composite formation as well as the resulting electrical and mechanical properties.
Materials
PEDOT:PSS
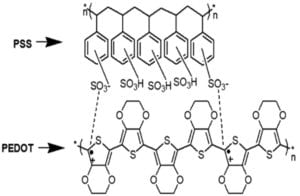
PEDOT is an excellent thermoelectric polymer that exhibits high electrical conductivity and low thermal conductivity. However, it is insoluble in water, which makes it difficult to process. As a result, PEDOT is typically polymerized with PSS serving as a counterion for PEDOT to stabilize and balance the charges. This produces an aqueous dispersion solution making it easier to deposit and form films. PEDOT:PSS is a conjugated polymer consisting of alternating single-double bonds along the carbon backbone resulting in overlapping p orbitals, which produce delocalized electrons for electrical conductivity (Figure 1). This polymer is brittle in practice due its complex conjugated structure.
chitosan
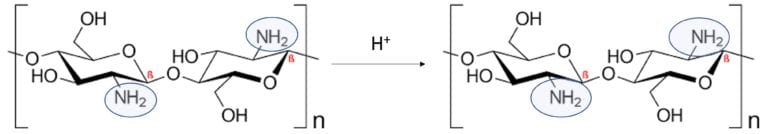
Chitosan is a biopolymer commonly extracted from crustaceans. It is an excellent candidate to mix with PEDOT:PSS due to its high mechanical flexibility and biodegradability. In addition, when chitosan is in an acidic medium the amino groups along the backbone become protonated and exhibit cationic behavior and was expected to have a doping effect for PEDOT:PSS to improve the electrical conductivity (Figure 2)
PVA
An alternative material was polyvinyl alcohol to increase the flexibility of PEDOT:PSS. It is a hydrophilic, water-soluble synthetic polymer with excellent film forming properties and high ductility.
Synthesis
Mixing with Chitosan
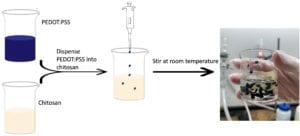
First, PEDOT:PSS was mixed with 6% (v/w) ethylene glycol, which acts as a dopant to increase the electrical conductivity. In a separate beaker, chitosan was dissolved in 5% (v/w) acetic acid and magnetic stirred for 24 hours. Once the chitosan was dissolved, amounts of PEDOT:PSS was dispensed into the chitosan solution and stirred at room temperature. Figure 3 shows the process of mixing the two polymer solutions and the resulting mixture. The solutions were not able to mix homogeneously and the PEDOT:PSS formed clumps in the chitosan solution.
mixing with pva
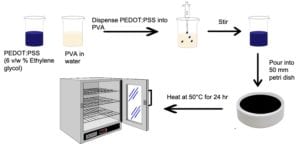
Mixing PEDOT:PSS with PVA follows the same process as mixing with chitosan. The PVA was magnetic stirred in water at 90°C for 4 hours, then cooled to room temperature and stirred for 24 hours. Appropriate amounts of PEDOT:PSS were dispensed into PVA to produce PEDOT:PSS/PVA weight ratios of 100:0, 50:50, 25:75, 10:90, and 0:100. After mixing, each solution was poured into 50 mm diameter plastic petri dishes and heated at 50°C for 24 hours in an oven. Figure 4 shows a schematic process of mixing PEDOT:PSS and PVA as well as the solution casting of PEDOT:PSS/PVA films.
Experimental Methods
Sample Preparation
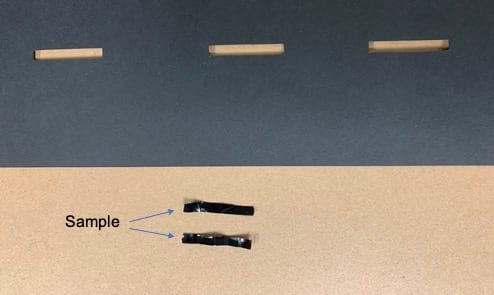
The solution cast films had a thickness of 0.035 mm, 0.0645 mm, 0.135 mm, 0.237 mm, and 0.418 mm for 100:0, 50:50, 25:75, 10:90, and 0:100 PEDOT:PSS/PVA, respectively (Figure 5). The thickness of the film samples were measured using a drop gauge. A 30 mm length by 5 mm width thick paper stencil was prepared to cut out film samples for electrical and mechanical testing (Figure 6).

2-Probe Multimeter test
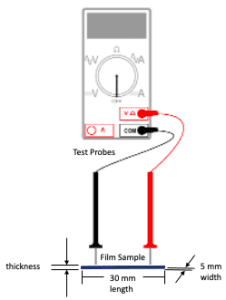
A 2-probe multimeter was used to measure the resistance of the polymer films (Figure 7). The electrical conductivity of the film samples were determined from the resistance measurements and sample dimensions.
Mini instron tensile test
The mechanical properties were investigated using a Mini Instron tensile test (Figure 8). The film samples were tested at a crosshead displacement rate of 5 mm/min until failure.

sem sample preparation
The film samples were fractured in a liquid nitrogen environment to simulate a natural fracture to ensure the microstructure is not distorted (Figure 9). The fractured samples were used for cross-sectional scanning electron microscopy (SEM) imaging (Figure 10).

Results & Discusion
electrical
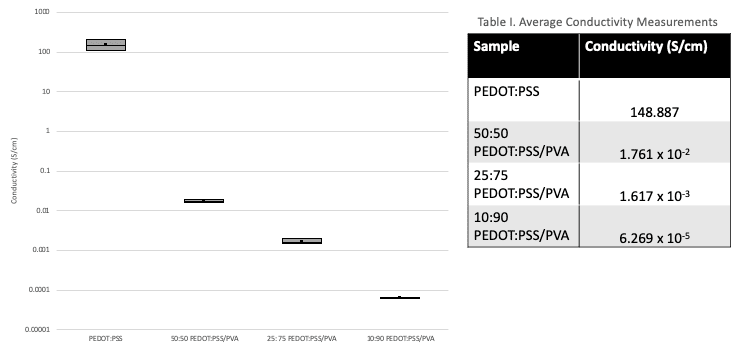
As expected, the electrical conductivity decreases with increasing amounts of PVA due to the insulating nature of PVA (Figure 11). Table I shows that PEDOT:PSS sample reaches a conductivity of 148.887 S/cm. In comparison, literature work has shown PEDOT:PSS doped with ethylene glycol to obtain conductivity values of 600-700 S/cm. It is suspected that the conductivity of the film samples were lower than expected due to the electrical contact resistance at the interface between the probe and film surface as well as the accuracy of the device. Using the four-point probe would have been better to conduct conductivity measurements. However, the equipment in lab is designed for inorganic materials. When the four-point probe was used to perform measurements, it pierced through the film samples.
Mechanical
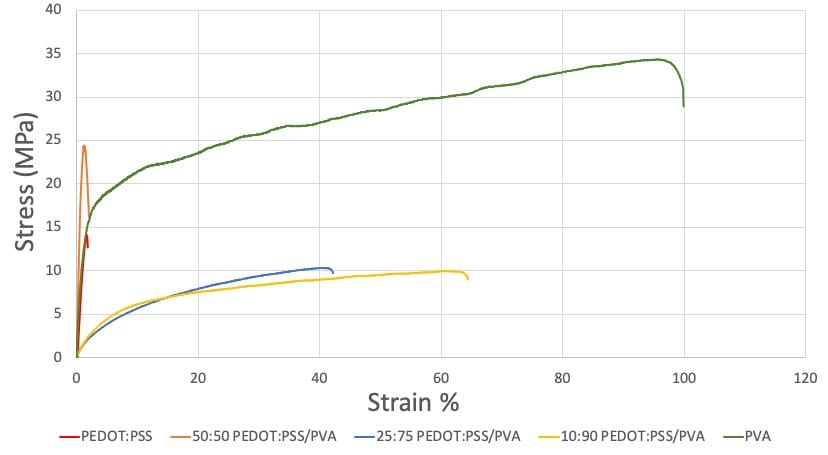
The stress-strain curves of the film samples show a significant increase in ductility and flexibility with increasing PVA content (Figure 12). The elongation to failure increased from 1.85% strain with PEDOT:PSS to 64.4% with 10:90 PEDOT:PSS/PVA. The tensile strength and elastic modulus shows no consistent trend as the content PVA increases among the film samples. Since each film weight ratio was only tested once, it is difficult to discuss these inconsistencies.
sem imaging
SEM images of the cross-sectional fractured surface of the films samples are depicted in Figure 13.
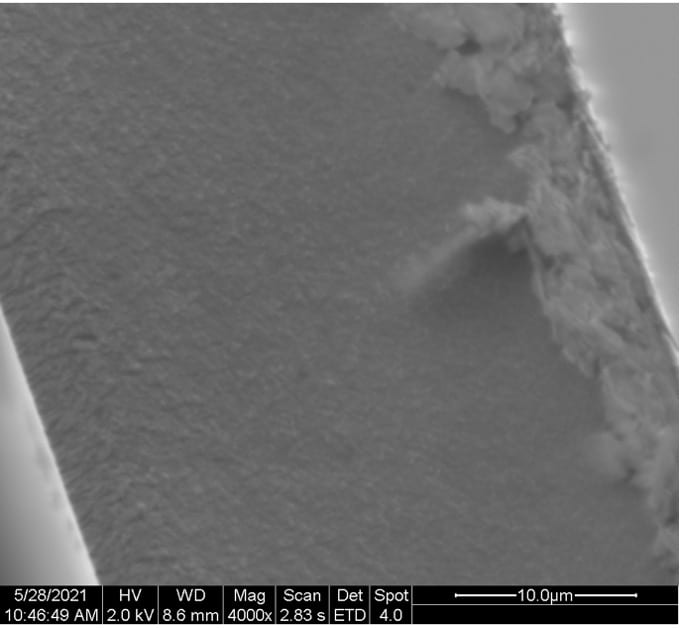
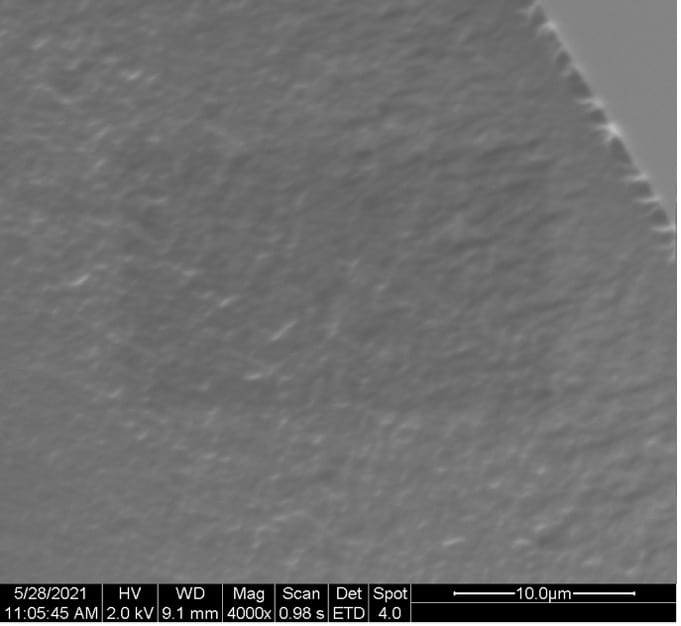
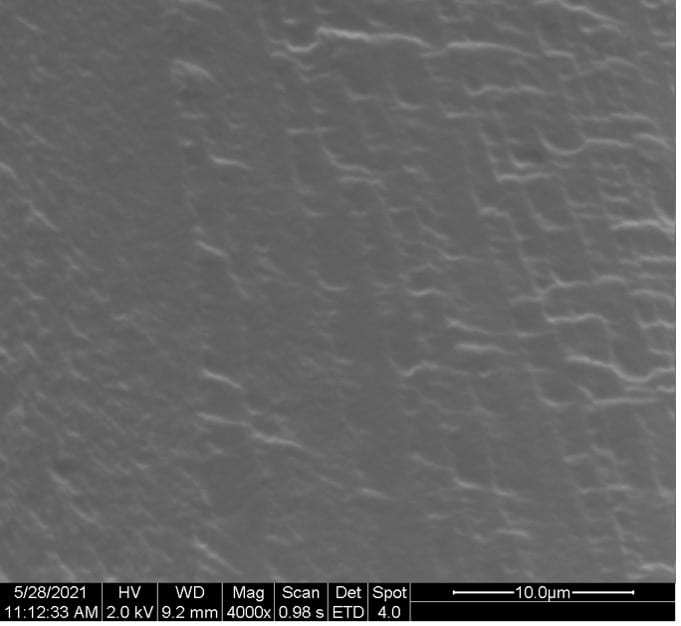
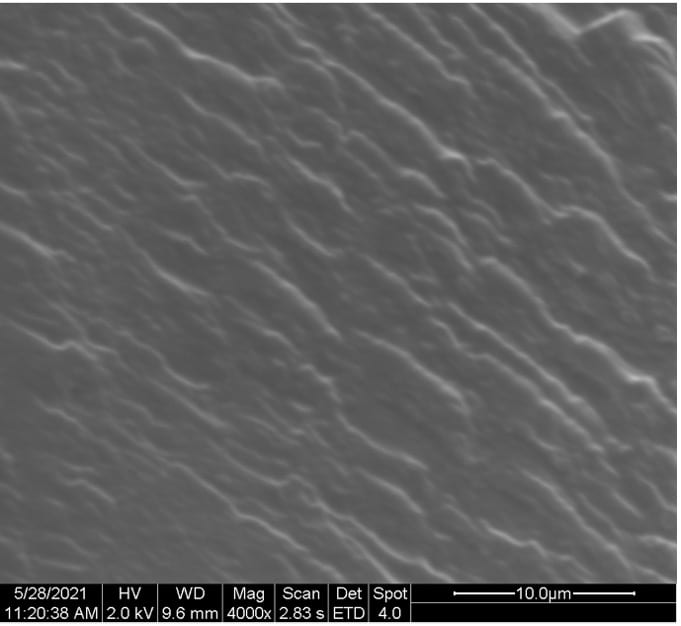
Figure 13. Cross-sectional SEM images of (a) 100:0, (b) 50:50, (c) 25:75, and (d) 10:90 PEDOT:PSS/PVA films.
Conclusion
1. Homogeneous, smooth, flexible, and stretchable thermoelectric films were successfully achieved using PEDOT:PSS and PVA with some loss in electrical conductivity
2. Conductivity measurements were lower than expected due to contact resistance between film sample and the test probes
3. Elongation significantly increased from 1.85% (PEDOT:PSS) to 64.4% (10:90 PEDOT:PSS/PVA)
Future Work
1. Investigate the miscibility of PEDOT:PSS and chitosan
2. Procure testing equipment to accurately measure electrical conductivity
3. Develop testing method to measure the Seebeck coefficient and thermal conductivity